In the evolving world of chemistry, few molecular combinations bridge organic synthesis, fuel innovation, and green chemistry as effectively as HCOOH CH2 H2O. This triad—comprising formic acid (HCOOH), a methylene group (CH2), and water (H2O)—represents more than a set of simple molecular fragments. It is a chemical synergy central to reactions involving carbon transfer, hydrogen release, and catalytic systems used in both industrial processes and sustainable technologies.
Understanding HCOOH CH2 H2O allows chemists to dive deeper into mechanisms of esterification, oxidation, hydrolysis, and even fuel cell development. This guide provides an expert-level breakdown of its molecular behavior, practical applications, and emerging potential in green science—optimized not only for academic clarity but also for cutting-edge industrial relevance.
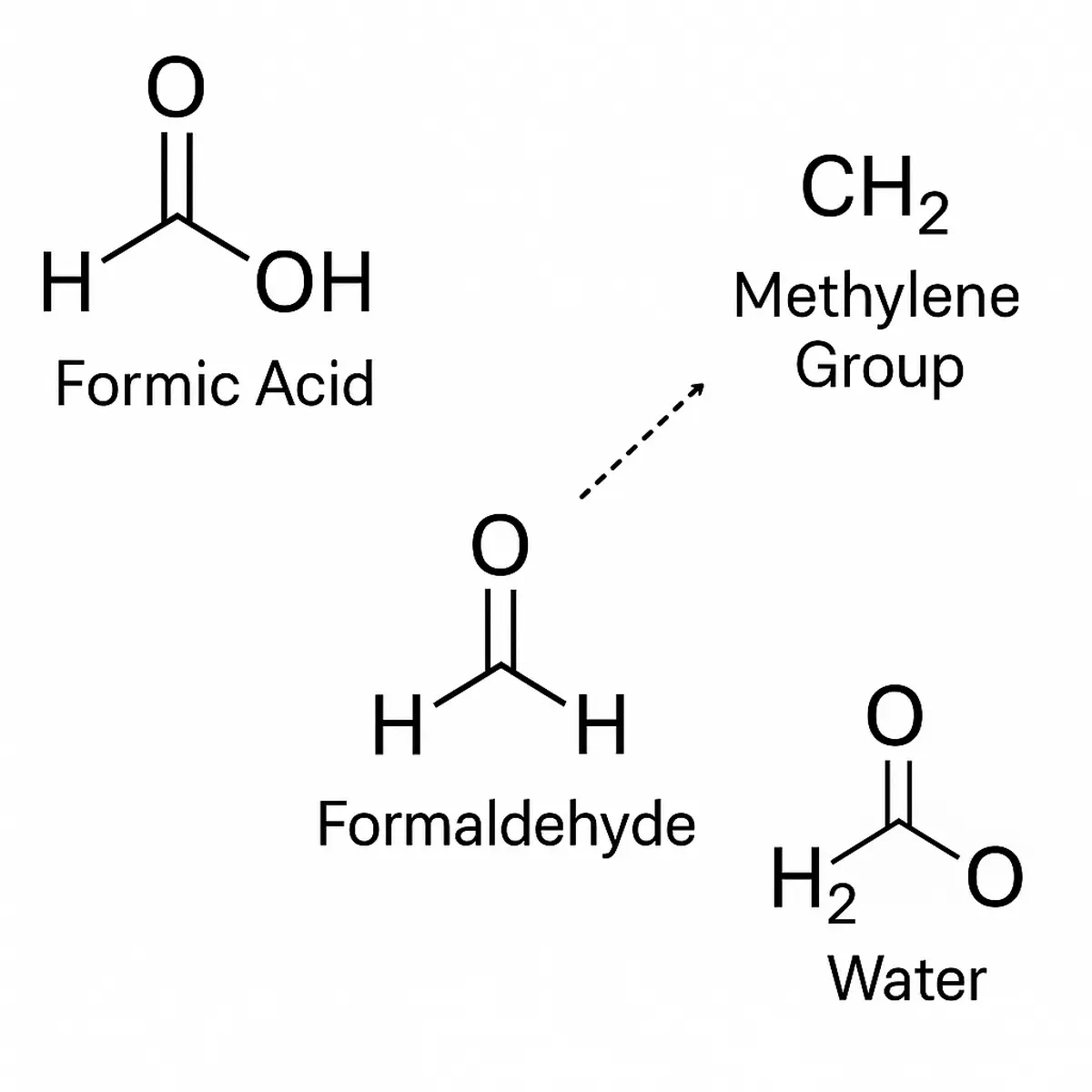
What is HCOOH CH2 H2O?
To comprehend the chemical and scientific importance of HCOOH CH2 H2O, it is essential to break it down into its molecular constituents:
- HCOOH – Formic acid, the simplest carboxylic acid (HCOOH), is a colorless, corrosive liquid with a pungent odor. It occurs naturally in ant venom and is industrially synthesized from methanol.
- CH2 – The methylene group (–CH₂–) is a key intermediary in organic reactions. In this context, it often refers to a reactive species such as formaldehyde (HCHO) or methylene bridge, which is central in various carbon-chain-forming reactions.
- H2O – Water, the universal solvent, is involved in almost all forms of reaction dynamics, from acid-base equilibria to complex hydration and hydrolysis reactions.
When these three entities are discussed together, especially under the shorthand HCOOH CH2 H2O, they frequently reference systems where formic acid is reacting or interacting with methylene-containing compounds in aqueous environments. This has profound implications in organic synthesis, fuel chemistry, and green solvent systems.
Molecular and Chemical Properties
Structural Overview
Formic acid (HCOOH) features a carboxylic acid group with a hydrogen atom directly bonded to the carbonyl carbon. This simple structure makes it highly reactive in acid-base and redox processes.
Methylene (CH2) is often represented as a bridging group or a formal radical. In reactions, it typically comes from precursors like formaldehyde (HCHO) or methylating agents.
Water (H2O) acts as both a solvent and a participant in hydrolysis, ion transport, and proton transfer.
Bonding and Interactions
In a reactive system involving HCOOH CH2 H2O:
- Hydrogen bonding dominates interactions, especially between formic acid and water.
- Electron pair donation and nucleophilic attack are common mechanisms, especially with the CH2 unit derived from formaldehyde or methanol.
- Proton transfer from formic acid enables activation of adjacent methylene groups.
Acid–Base Behavior
Formic acid is a weak acid (pKa ≈ 3.75), which dissociates in water:
HCOOH⇌H++HCOO−\text{HCOOH} ⇌ \text{H}^+ + \text{HCOO}^-HCOOH⇌H++HCOO−
This equilibrium plays a role in titration analysis and in buffer systems.
Reactions Involving HCOOH CH2 H2O
1. Esterification
Formic acid readily reacts with alcohols in acidic conditions to form esters:
HCOOH+CH3OH⇌HCOOCH3+H2O\text{HCOOH} + \text{CH}_3\text{OH} ⇌ \text{HCOOCH}_3 + \text{H}_2\text{O}HCOOH+CH3OH⇌HCOOCH3+H2O
In the presence of a methylene compound like methanol (CH3OH), this equilibrium reaction yields methyl formate—a volatile solvent and precursor in pharmaceuticals.
2. Hydrolysis and Hydration
Formaldehyde (CH2O) derived from the methylene group undergoes hydration:
CH2O+H2O⇌CH2(OH)2\text{CH}_2\text{O} + \text{H}_2\text{O} ⇌ \text{CH}_2(\text{OH})_2CH2O+H2O⇌CH2(OH)2
This gem-diol intermediate can further react with formic acid, leading to formylation reactions, or undergo oxidation.
3. Oxidation and Reduction
Formic acid serves as a reducing agent, especially under catalytic conditions:
HCOOH→CO2+H2\text{HCOOH} → \text{CO}_2 + \text{H}_2HCOOH→CO2+H2
This decomposition, catalyzed by palladium or platinum, is key in hydrogen release for fuel cells.
In the presence of methylene compounds, a variety of oxidation states and carbon–carbon bond formations are possible.
Laboratory and Industrial Applications
Fuel Cell Technology
Formic acid–based fuel cells (DFAFCs) utilize HCOOH as a liquid hydrogen carrier. Combined with water and methanol/formaldehyde, it serves as a compact, storable, and efficient hydrogen source.
Reaction:
HCOOH→CO2+H2\text{HCOOH} → \text{CO}_2 + \text{H}_2HCOOH→CO2+H2
These systems are being explored in portable energy devices and as sustainable alternatives to hydrogen gas tanks.
Organic Synthesis
The HCOOH CH2 H2O system is central in:
- Methylation reactions: using methylene donors like formaldehyde or dimethyl sulfate.
- Polymer formation: CH2 bridges are key in phenol–formaldehyde resins.
- Preservatives and disinfectants: formic acid acts as both a microbial inhibitor and chemical intermediate.
Real-World Example
Leather tanning: Formic acid is used to adjust pH and open collagen structures. When mixed with formaldehyde in water, it modifies protein chains, improving dye absorption and flexibility.
Role in Analytical and Green Chemistry
Analytical Chemistry
Formic acid in titration: Due to its known acidity, HCOOH is a primary standard in acid–base titrations.
Chromatography: As a mobile phase modifier in liquid chromatography, HCOOH helps adjust polarity and pH, improving peak resolution.
Spectroscopy: Formic acid and its derivatives exhibit strong IR absorbance (C=O stretch near 1730 cm⁻¹), enabling trace analysis in mixtures.
Green Chemistry and Sustainability
HCOOH CH2 H2O systems are:
- Biodegradable and low-VOC compared to petroleum-based solvents.
- Used in bio-based ester production with minimal environmental impact.
- Part of catalytic systems for carbon-neutral fuel cycles.
Catalytic example:
HCOOH+CH2O→Pt/CCH3OH\text{HCOOH} + \text{CH}_2\text{O} \xrightarrow{\text{Pt/C}} \text{CH}_3\text{OH}HCOOH+CH2OPt/CCH3OH
This reaction forms methanol—a key industrial fuel and chemical feedstock.
Safety and Environmental Considerations
Handling Formic Acid
- Corrosive to skin and mucous membranes.
- Should be handled with PPE, including gloves and goggles.
- Requires storage in corrosion-resistant containers.
VOC and Disposal
Although formic acid is a low-VOC solvent, care must be taken during decomposition reactions, as CO₂ release and pressure buildup can occur.
Waste Disposal:
- Neutralization with sodium bicarbonate before drainage.
- Oxidative treatment for methylene-containing wastes.
Laboratory Protocols
- Use in fume hoods.
- Avoid heating closed systems with HCOOH due to CO₂ buildup.
- Store away from oxidizers and alkalis.
Future Research Directions
Hydrogen-Based Fuel Systems
Research is intensifying into formic acid as a hydrogen source for on-demand hydrogen generation. Systems involving HCOOH CH2 H2O are promising due to low toxicity and compact energy density.
Green Solvents and Catalysis
Efforts are ongoing to replace toxic reagents with:
- Aqueous formic acid–methylene systems for C1 chemistry.
- Bio-catalyzed transformations using engineered enzymes.
Smart Catalysts
Development of nano-catalysts (Pt, Pd, Au) for:
- Faster decomposition of HCOOH.
- Selective oxidation/reduction involving CH2 bridges.
These could revolutionize carbon-neutral fuel technologies and on-site hydrogen production.
Conclusion
HCOOH CH2 H2O represents a powerful trinity in modern chemistry. It spans the fields of organic synthesis, industrial production, green chemistry, and energy storage. From creating methyl esters and processing leather to generating clean hydrogen for fuel cells, its relevance is expansive and growing.
As research pushes the boundaries of eco-innovation and carbon efficiency, this combination of formic acid, methylene intermediates, and water will remain pivotal. Understanding its chemistry unlocks new potential for sustainable industrial practices, making it an essential focus for chemists, engineers, and environmental scientists alike.

Pingback: How DGH A Became a Cornerstone Code Across Healthcare, Education, and AI